Nitinol collection bag
designed to capture
and remove clot
Atraumatic coring element separates
and extracts wall adherent clot
from the vessel
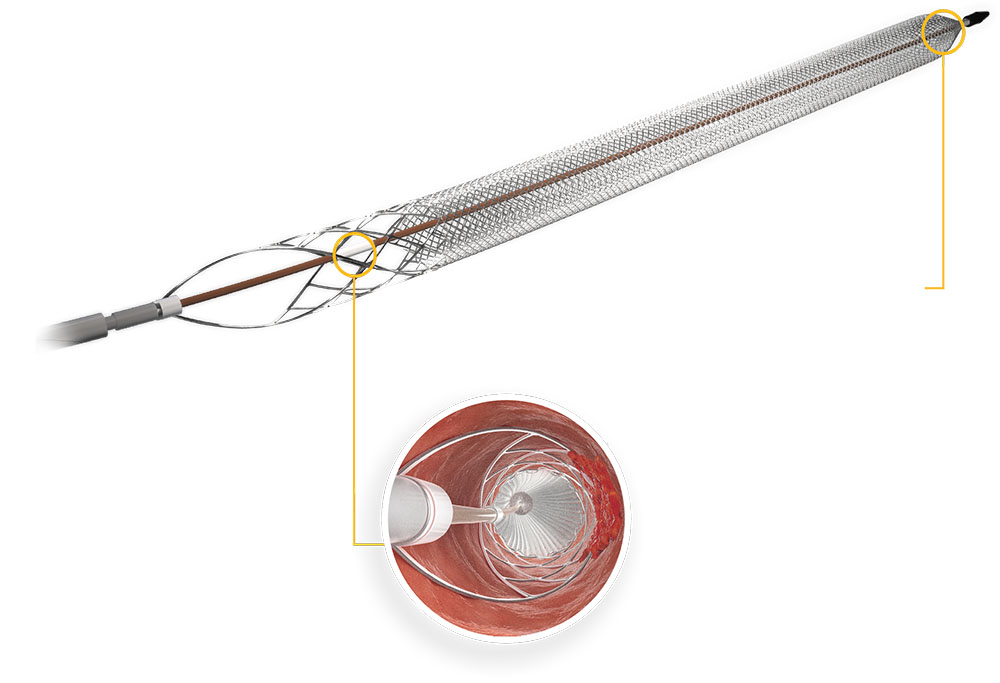
ClotTriever catheter:
- The ClotTriever catheter gains complete wall
apposition in vessels 6-16 mm for the collection
and removal of clot.

30%
shorter collection bag
HyperClear™
Technology
The latest in surface
treatment innovation
†compared to 1st generation ClotTriever BOLD
ClotTriever BOLD catheter:
- The next generation ClotTriever BOLD catheter
features a shortened collection bag† and
HyperClear™ technology, delivering the same
trusted performance for the treatment of DVT with
faster procedure times.†
-
HyperClear Techology is the latest in surface
treatment innovation designed to reduce clot
adhesion within the collection bag for improved bag
clearing and procedure times.†
10 cm collection bag
Shorter bag for easier positioning of the catheter
10 – 28 mm vessel treatment range
Enables coring and collection of thrombus with
full wall apposition
105 cm effective length
For extended range of treatment
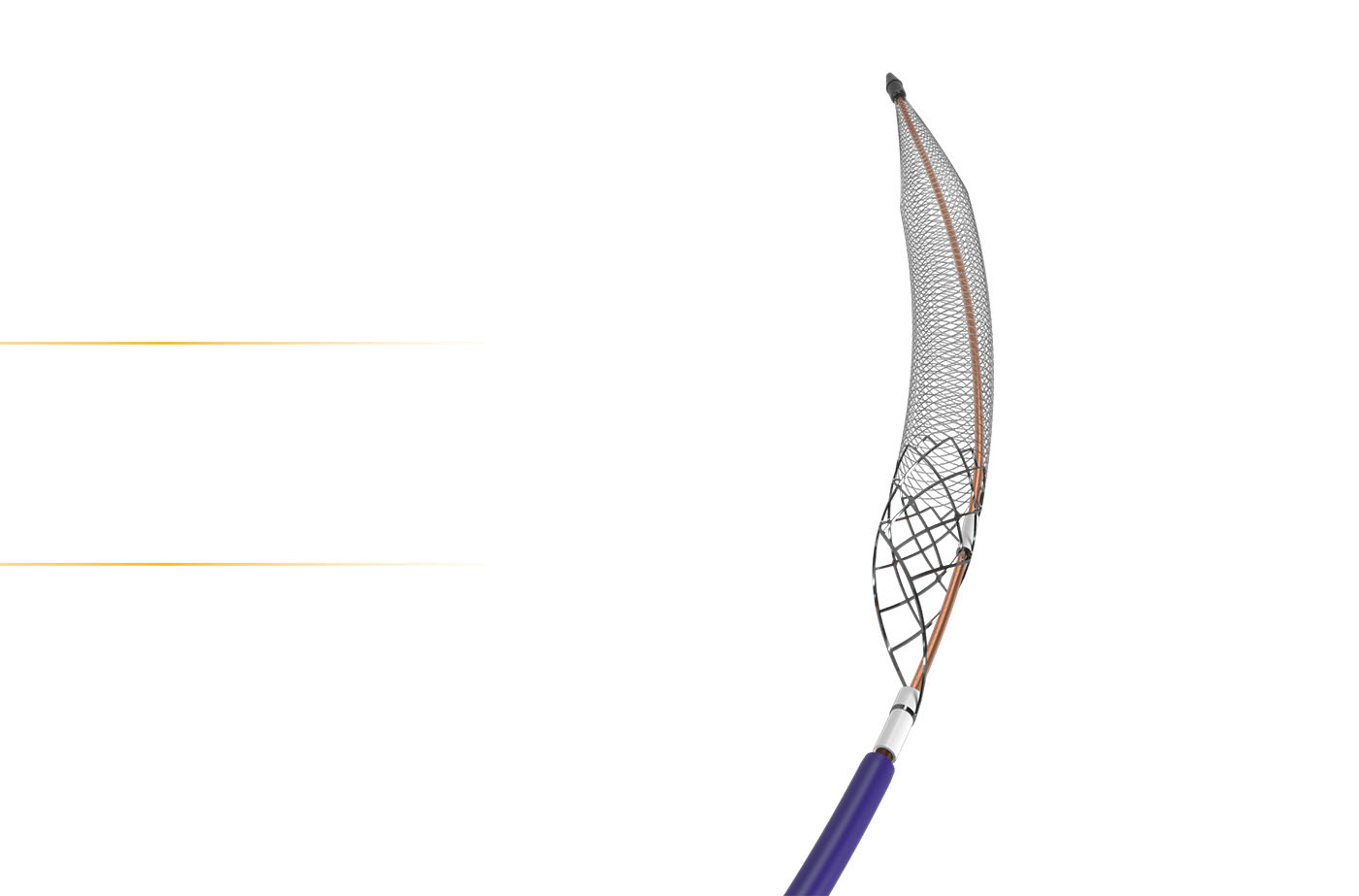
ClotTriever XL catheter:
- A larger device for the largest vein.
Purpose-built design for efficient clot removal
in the vena cava.

Self-expanding nitinol funnel designed
for 360º wall apposition for optimal
clot extraction
Single-hand operation hemostasis valve
to minimize blood loss during insertion
and removal of devices
ClotTriever sheath:
- The ClotTriever sheath is specifically
designed for optimal clot removal
with minimal blood loss.





