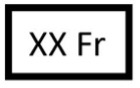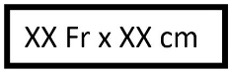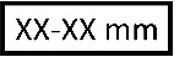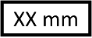| ISO 15223-1 Symbols: Medical devices – Symbols to be used with information to be supplied by the manufacturer |
|---|

|
Manufacturer |
Indicates the medical device manufacturer. |
5.1.1 |

|
Authorized representative |
Indicates the authorized representative in the identified country or jurisdiction. |
5.1.2 |

|
Authorized representative for Switzerland |
Indicates the authorized representative in Switzerland. |
5.1.2; ISO 20417 6.1.2 |

|
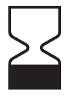
Date of manufacture |
Indicates the date when the medical device was manufactured. |
5.1.3 |
|
Use-by date |
Indicates the date after which the medical device is not to be used. |
5.1.4 |

|
Batch code |
Indicates the manufacturer’s batch code so that the batch or lot can be identified. |
5.1.5 |

|
Catalogue number |
Indicates the manufacturer’s catalogue number so that the medical device can be identified. |
5.1.6 |

|
Importer |
Indicates the entity importing the medical device into the locale. |
5.1.8 |

|
Country of manufacture |
To identify the country of manufacture of products. |
5.1.11 |

|
Sterilized using ethylene oxide |
Indicates a medical device that has been sterilized using ethylene oxide. |
5.2.3 |

|
Do not resterilize |
Indicates a medical device that is not to be resterilized. |
5.2.6 |

|
Do not use if package is damaged; consult IFU |
Indicates a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use. |
5.2.8 |
|
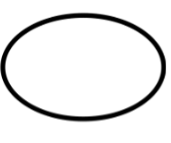
Single sterile barrier system |
Indicates a single sterile barrier system. |
5.2.11 |
|
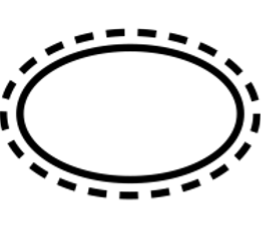
Single sterile barrier system with protective packaging outside |
Indicates a single sterile barrier system with protective packaging outside. |
5.2.14 |
|
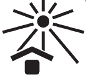
Keep away from sunlight |
Indicates a medical device that needs protection from light sources. |
5.3.2 |

|
Keep dry |
Indicates a medical device that needs to be protected from moisture. |
5.3.4 |

|
Do not re-use |
Indicates a medical device that is intended for one use only. |
5.4.2 |

|
Consult instructions for use / electronic IFU |
Indicates the need for the user to consult the instructions for use. |
5.4.3 |

|
Caution |
Indicates that caution is necessary or operator awareness/action is needed to avoid undesirable consequences. |
5.4.4 |
|
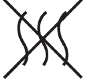
Non-pyrogenic |
Indicates a medical device that is non-pyrogenic. |
5.6.3 |

|
Medical devices |
Indicates the item is a medical device. |
5.7.7 |

|
Patient identification |
Indicates the identification data of the patient. |
5.7.3 |

|
Patient information website |
Indicates a website where a patient can obtain additional information on the medical product. |
5.7.4 |

|
Health care center or doctor |
Indicates the address of the health care center or doctor where medical information about the patient may be found. |
5.7.5 |

|
Date |
Indicates the date that information was entered or a medical procedure took place. |
5.7.6 |

|
Unique Device Identifier |
Indicates a carrier that contains unique device identifier information. |
5.7.10 |
| ASTM F2503: The labeling of devices as MR Safe, MR Conditional, and MT Unsafe |
|---|
-unsafe.png)
|
Magnetic Resonance (MR) Unsafe |
An item which poses unacceptable risks to the patient, medical staff, or other persons within the MR environment. |
ASTM F2503 |

|
Magnetic Resonance (MR) Conditional |
An item demonstrates no hazard in the MR environment, but only when prescribed conditions for safe use are adhered |
ASTM F2503 |
| US Code of Federal Regulations, Title 21, Part 801.109(b)(1): Prescription devices |
|---|
|
|
Prescription device |
Caution: United States Federal Law restricts this device to sale by or on the order of a physician. |
21 CFR 801.109(b)(1) |
| Symbols for Certification and Compliance |
|---|
|
|
European Conformity |
Indicates conformity to Regulation (EU) 2017/745 and that the product is authorized for sale in EU countries.
May be accompanied by Notified Body ID (e.g., 0297 DQS, 0459 GMED).
|
— |
| Symbols Not from Standards |
|---|
|
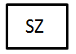
Size |
Overall size of the medical device. |
— |
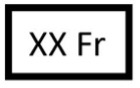
|
Catheter / delivery catheter size |
French size (Fr) of the catheter or delivery catheter. |
— |
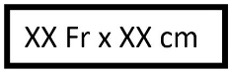
|
Sheath / catheter size and length |
French size and effective length of sheath or catheter. |
— |
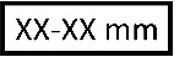
|
Vessel Diameter |
Target vessel diameter for device compatibility. |
— |
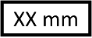
|
Coring Element Diameter |
Diameter of coring element. |
— |

|
French size & Coring Element Diameter |
French size of the catheter/delivery catheter and diameter of coring element. |
— |

|
French size & metric diameter |
French size of the catheter/delivery catheter and metric diameter. |
— |

|
French size & vessel diameter |
French size of the catheter/delivery catheter and vessel diameter. |
— |

|
French size & syringe capacity |
French size of catheter and capacity of the syringe. |
— |
|
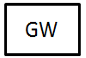
Recommended Guidewire Size |
Indicates the recommended guidewire size needed to use the medical device. |
— |

Or

|
Contents and/or Quantity |
Contents and/or quantity of medical device. |
— |


























-unsafe.png)